My son
is a joy. And he’s probably the healthiest kid with Hunter Syndrome you’ll ever meet.
Right now anyway.
He won’t be soon if Blue Cross / Blue Shield of Tennessee has their way.
For the fourth time in the last 18 months, I’ve received a claim denial from Blue Cross / Blue Shield of Tennessee for my son Case’s medically necessary drug Elaprase (keep reading to see the denials and their reasons for them). Elaprase, or idursulfase, is a manufactured form of the enzyme iduronate-2 sulfatase – the enzyme Case’s body is lacking. The missing enzyme is caused by a genetic mutation resulting in his disease, Hunter Syndrome or Mucopolysaccharidosis / MPS II.
[su_note note_color=”#f4831f”]CLICK HERE to SIGN and SHARE this petition to urge BCBS of Tennessee to rescind their new policy regarding Idursulfase coverage
[/su_note]
Elaprase is the only FDA-approved drug for Hunter Syndrome. It is not a cure. But by giving the enzyme to kids with Hunter Syndrome, it has been shown, in clinical trials and in my nine years of experience with Hunter Syndrome, to absolutely and unequivocally slow down and in some cases, halt and reverse the physical effects of the disease.
Case receives a weekly infusion of Elaprase over 4 hours. He has a port-a-cath through which he receives the drug.
It is an expensive drug.
But it’s not the most expensive drug either.[1] AND it is also the standard of care for a child diagnosed with Hunter Syndrome in the United States. No physician would argue otherwise.
It is the standard of care for a newborn with Hunter Syndrome. It is the standard of care for a cognitively impaired child with Hunter Syndrome. It is the standard of care for an attenuated (non-cognitively impaired) child or adult with Hunter Syndrome.
In other countries or in some cases, patients may elect a bone marrow transplant or because of certain risks or preferences, choose not to receive these weekly infusions, but those are the exceptions.
But because of that drug, Case is walking. He is running. His heart valves are stable. And because he is on that drug, he was able to qualify for a clinical trial at the age of 3 1/2 where a more concentrated form of the drug (Idursulfase-IT) was put into his brain.
He’s received that new drug in his brain for 7 years, and like the positive physical effects of Elaprase, this new drug has dramatically halted and even reversed some of the brain decline in his disease. He receives it every four weeks via a spinal tap and since it is a clinical trial, the drug company currently pays for those expenses. But in order to continue, he has to receive the weekly infusion of Elaprase as well.
Neither of those drugs are cures for Hunter Syndrome. But then again, neither are drugs for diabetes, heart disease, ADHD, or depression.
The nature of medicine, and of scientific research, is that we treat symptoms of diseases the best way medicine allows until the science catches up and develops a better treatment, or in that rare case, finds a cure.
But most treatments simply slow down or halt progression or treat symptoms of diseases until that elusive cure can be found.
In fact, at Project Alive, we are preparing to begin clinical trials for that hopeful cure for Hunter Syndrome – gene therapy, but we need to keep our boys alive and healthy long enough to get there. Keeping them alive and relatively healthy is Elaprase – the standard of care for boys with Hunter Syndrome.
But Blue Cross / Blue Shield of Tennessee doesn’t want to pay for it.
[su_note note_color=”#f4831f”]CLICK HERE to SIGN and SHARE this petition to urge BCBS of Tennessee to rescind their new policy regarding Idursulfase coverage
[/su_note]
I carefully selected our insurance plan.

When your child’s life is sustained by a costly medicine, it is the first thing in mind when you compare and select your health insurance.
When I chose our current insurance plan in September 2016, I reviewed all of the documentation, including which drugs required pre-authorization. Elaprase DID NOT require pre-authorization when we enrolled in our plan.
But unbeknownst to anyone with knowledge relevant to such a decision, on December 1, 2016, BCBS of Tennessee adopted a new medical policy for Elaprase, not only requiring pre-authorization, but also very specific medical criteria for receiving the drug.
That new policy was not sent to us, although my personal knowledge within the Hunter Syndrome community suggests that my son is the only BCBS of Tennessee subscriber receiving Elaprase.
So when I received my first retroactive denial letter for coverage of Elaprase in the first half of 2017, 8 years after my son first started receiving Elaprase, I had to backtrack to find this policy, figure out what to do, and how to keep my son alive. As it turns out, no medical provider sought pre-authorization for Elaprase because, shocker, this entirely new policy went into effect at the end of the calendar year, unbeknownst to me, right before I ordered a new shipment.
I called BCBS of Tennessee and went through the whole scenario of the new policy after we enrolled in the plan, the fact that my son has been on this medicine for 8 years, and that no one notified us of the new policy so that we could seek pre-authorization. Their response:
- They don’t need to notify me of any pre-authorization or medical policy changes for my son’s life-sustaining medication.
- Physicians, providers, pharmacies are responsible for complying with these policies, not me.
- Since the denial was retroactive and my child had already received the drug, why did it matter to me?
So fine. The specialty pharmacy absorbed the cost of the drug for January – March 2017 until they could circle back to Case’s physician and get pre-authorization.
BCBS of Tennessee saved itself $150,000. Yes, that’s the cost for January – March. So you can see why they’re incentivized to adopt policies that allow them to retroactively deny a bill without notifying the patient being saved by that drug. Hope someone got a bonus.
But in any event, I was relieved to have it behind us and that they weren’t triggering these other provisions in their adopted-under-cover-of-Christmas Elaprase medical policy.
Merry Christmas, a little early.
Near the end of 2017, I opened up my mail to a new denial.

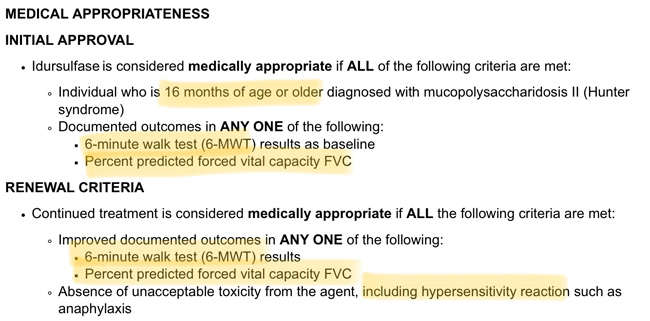
BCBS of Tennessee denied Case’s Elaprase based on the fact that he never had an initial 6-minute walk test (6-MWT) or Percent Predicted Forced Vital Capacity (measured through a test called spirometry) and has not now shown improvement in those tests.
The applicable portion of their recently enacted policy, which can be found in its entirety HERE:
And now, every few months there is a new denial, going in circles from utilization management to someone else and then back again. Sometimes the claims are paid, and sometimes they’re not.
But here’s the rub.
My child can’t meet ANY of the criteria in BCBS-TN’s policy, and neither can most children with Hunter Syndrome.
[su_note note_color=”#f4831f”]CLICK HERE to SIGN and SHARE this petition to urge BCBS of Tennessee to rescind their new policy regarding Idursulfase coverage
[/su_note]
A child with the cognitively impaired form of Hunter Syndrome (at least 2/3 of those affected by the disease), with the rare exception, can’t do either a 6-MWT or spirometry, even those like Case who are doing well cognitively. Because of their cognitive impairment (which I have coined as a combination of features of ADHD, autism, OCD, and sensory processing disorder), they generally can’t attend to walking for 6 minutes straight, and any declines or improvements noted over time would equally likely be attributable to distraction as they would be to walking (in)ability, improvement or decline.
With respect to spirometry, as described by Mayo Clinic, carries the following expectations:
- You’ll likely be seated during the test.
- A clip will be placed on your nose to keep your nostrils closed.
- You will take a deep breath and breathe out as hard as you can for several seconds into the tube. It’s important that your lips create a seal around the tube, so that no air leaks out.
- You’ll need to do the test at least three times to make sure your results are relatively consistent. If there is too much variation among the three outcomes, you may need to repeat the test again. The highest value among three close test results is used as the final result.
- The entire process usually takes less than 15 minutes.
Who would expect a child with the cognitive and behavioral impairments I described as being able to complete this process? They can’t. Neither can most children under the chronological or cognitive age of 6 years old.

And by requiring documented continued improvement in one of those tests, it shows a lack of a basic understanding of the disease. Hunter Syndrome is a progressive disease that has been shown to cause multi-system decline over time. Treatments which stabilize these symptoms or even slow down the decline are effective ones. It is not even remotely appropriate to expect continued improvement over time in a progressive disease, I don’t care what treatment you’re using.
So by requiring those tests to initiate or continue on Elaprase, BCBS of Tennessee is effectively eliminating its use in the vast majority of kids with Hunter Syndrome. And by requiring continued improvement over time, BCBS of Tennessee seeks to effectively rid itself of covering almost all patients with Hunter Syndrome.
Since I believe Case to be the only Hunter Syndrome patient covered by BCBS of Tennessee right now, they will at least have effectively saved themselves the cost of him. And for Case, should he be unable to receive Elaprase, he also could no longer receive his clinical trial drug either. No trial drug = rapid brain decline = death.
BCBS of Tennessee, as with the first denial, by denying the bill retroactively and under a policy that does not meet the standard of care for the disease, is counting on the specialty pharmacy and/or the drug company to eat the cost, at least for awhile, to save money. Had they actually spoken with an expert in Hunter Syndrome, or any geneticist that treats a patient with Hunter Syndrome, or a lay expert in Hunter Syndrome, such as myself, they would know that.
But instead, several times in 2017 and 2018, I, my son’s doctors, the specialty pharmacy, and the drug company, have been forced to call, sit on the phone for hours (I’ve written about that before), be transferred back and forth, not have our calls returned, call back again, talk to people who know NOTHING about Hunter Syndrome, and in general make a fuss so a company can correct its own error, made without telling anyone to whom it would apply (us) and after insurance had already been selected for the year.
And here’s the interesting part… they’re not even applying their own policy consistently to deny my son this medicine. Sometimes they pay, sometimes they don’t. Instead, they’re applying it retroactively (to make sure he gets the drug), so that their annual cost just goes down. They know he needs the drug and it’s medically necessary. They’re just using a system to reduce their costs.
Could I appeal and ask for them to make an exception here? SURE
Could I let someone else handle this? SURE
But this is a BAD policy. It shows a clear lack of understanding of the disease, its symptoms, and the implications of its policy. These two tests (6-MWT and spirometry) were in the original Elaprase clinical trials, which ended well before FDA approval in 2006 and only included non-cognitively impaired patients, but a vast majority of data, including real world evidence (RWE) has emerged and been published in peer reviewed journals in the last 11 years since 2006, the year Elaprase was approved, and this policy is not in alignment with the standard of care. Why hasn’t a clinician challenged it already? Because, like I said, my son is likely the only patient to whom this policy applies. And there are no local clinical experts in the disease – that would be me, check my credentials, which include recognized expertise in Hunter Syndrome, just lacking the MD. So….
I am calling on BCBS of Tennessee to rescind this policy.
And if BCBS of Tennessee and other critics of the high cost of orphan drugs are really interested in saving money in the big picture, they’ll want to read Part 2 of this series: How the Health Care System Can (Really) Save Money
[su_note note_color=”#f4831f”]
UPDATE: After initially writing this piece, I’ve since received additional denials based on a peer-to-peer medical review between our physician and BCBSTN. One denial states:
The information provided for Elaprase does not meet the BlueCross BlueShield of Tennessee’s Idursulfase Medical Policy for Hunter syndrome. The guideline requires certain lab or pathology (blood) tests to be performed. The required lab/pathology test for 6-minute walk test (6-MWT) or percent predicted forced vital capacity (FVC) (a lung function test) results as baseline was not obtained prior to the use of Elaprase. The requirements support the use of Elaprase for Hunter syndrome when a 6-MWT or FVC is obtained. Therefore, the requested medication does not meet the medical necessity criteria.
[su_note note_color=”#f4831f”]This is Part 1 of a 3-part series on orphan drug pricing and access to treatment. Coming up:
Part 2: How the Health Care System Can (Really) Save Money
Part 3: Who is Behind the Attacks on Orphan Drugs[/su_note]
———————————-
[1] America’s Most Expensive Prescription Drugs, CNBC (05/30/2017); The 7 Most Expensive Prescription Drugs In The World, The Motley Fool (4/18/2017); The New Top 10 Most Expensive Drugs on the Planet, Endpoint News (04/28/2017). Interestingly, the first list is almost completely different than the last two lists. The CNBC list comes from an average 30-day supply and mostly consists of drugs not taken in perpetuity, whereas the Motley Fool and Endpoint News lists are populated more by drugs taken in perpetuity with high annual costs. The monthly (>$40,000) or annual (>$500,000) cost for my son’s dose of Elaprase would otherwise make every list if one considered his dose to be the average dose.

7 responses to “Blue Cross Blue Shield of TN Wants My Son to Die”
Melissa, I would love to email you a copy of one of andrews bills for elaprase. His is $62,640 a week just for the elaprase, then $229 for the cost of them accessing him for the first hour and $138 for every journey after that. Let me know if you would like a copy for your fight against this.
Having gone through fighting with an insurance company over medical decisions for my son- I pray for you Mama! Continue appealing, after your 3rd (I think) appeal you get to go before a “board” and present your case to them. Although a different case, my exact words to them at the end of my entire argument was that by continuing to deny my request for his birth at an “out of network” hospital every single one of them were essentially signing my unborn sons death certificate. It’s so sad that serious life changing medical decisions are made by people who have no clue 😩
😤
As a Pediatric nurse for special needs children this is terrible how the Insurance Companies dictate what these children need when they are clueless. They should be in this situation to see what these special children go thru every day if their lives.
They do this because if it were any other progressive disease that a lot of people have it would be plastered all over the front page! My husband has Progressive MS and the drugs he is on will not cure it and he won’t get better but he has to have them to live. How is treating Hunter Syndrome different? It’s so rare that the outrage isn’t there when Case is the only one in Tennessee that has it. There is a place in hell for these people who have denied him. It isn’t the big company!!! There are people making these decisions. Shame on BCBS of Tennessee!$
Sorry to hear you are having such a difficult time with BCBC of Tennessee. Have you tried contacting the Hunter Syndrome Foundation or MPS-II Society for help. Parents Groups for Hunter Syndrome can sometimes help. Even as an adult I had a difficult time doing my first PFT – you should be given additional time and practice for your son to get results needed for a PFT.
Good luck. As a parent you go through difficult times just dealing with a sick child, doctor appointments, school issues that you don’t need to have to fight the insurance companies.
Mother of a Cystic Fibrosis young adult who passed away at 26. There is no cure for CF but treatments help them live longer.
Hi Eileen! I love when is Rare disease families stick together. I’m so sorry for your own loss.
Yes, I know the folks at both of those organizations, but I run Project Alive, a leading Hunter Syndrome research and advocacy foundation and do lots of advocacy work myself. This effort is not only for my son, but for all of those kids who might come after him in TN and would otherwise not get access to Idursulfase if they had BCBS TN as their insurer.